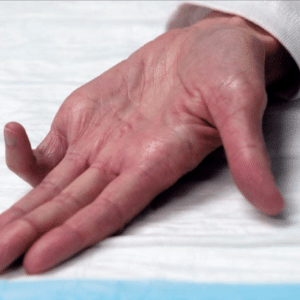

The clinical use of this biochemical agent involves an injection performed in the office to digest the cord collagen. Patients who have contracture at the MCP (the knuckle) or at the PIP (the first joint) from Dupuytren’s fibrosis are candidates for the injection therapy. The fibrosis in the palm is not as responsive to injection therapy and may require excisional surgery using tourniquet anesthesia. Patients who have the finger contracture can be injected monthly up to three times. The patient is seen the next day for a mobilization maneuver to hopefully break the cord. Tendon rupture is a small risk of the injection, because it contains high collagen content and is in close proximity to the fibrotic cord in the finger. Dr. Paul Vanek is credentialed in the safe use of Xiaflex™ collagenase injection. The procedure is not cosmetic.
When you sign up for our mailing list, you'll receive a special offer.

Dr. Vanek will listen to you and “hold your hand” through your entire process, and he has taught his entire staff to do the same. He understands and respects your privacy and sensitivity. His professional but familial approach will quickly help you become very comfortable with him and with how he will help change your life.
Please complete this form, including any procedures you may be interested in. We will contact you as soon as possible by email, phone, or text (as you specify)!